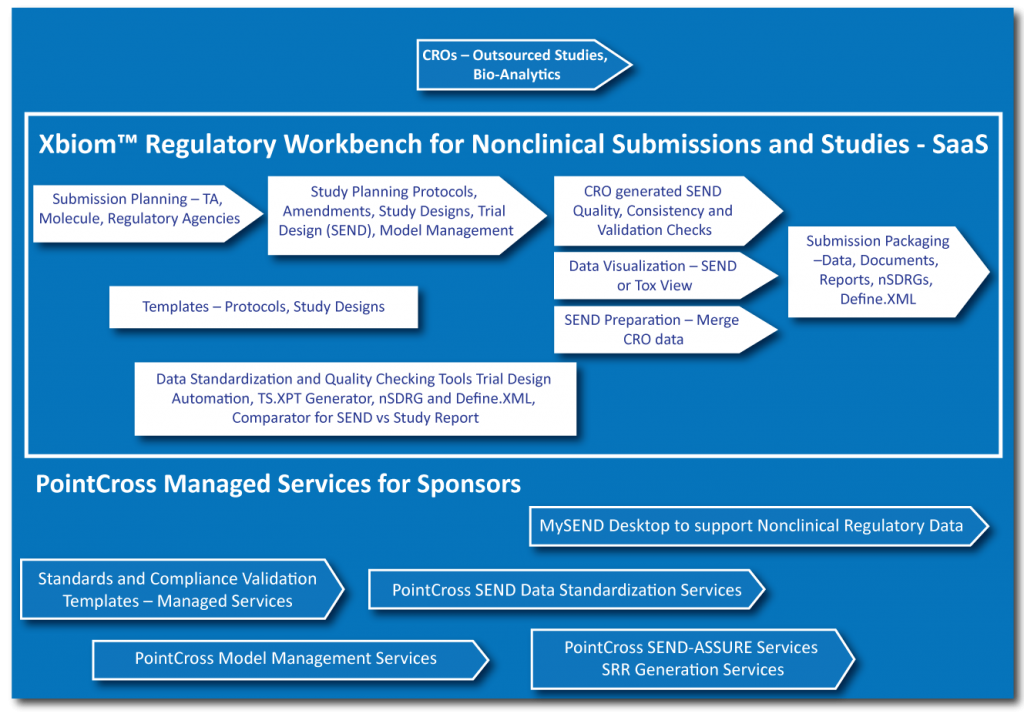
Workflows for planning submissions and studies . Seamless encrypted interactions with CROs . Define Study and Trial Design Models . Merge SEND study components and validate SEND studies provided by CROs . QC Dashboards, Quality Tools and Validators
Why should Sponsors consider Xbiom?
- Nonclinical studies are being outsourced to CROs and Bio-Analytics Service companies but Sponsors:
- Own and execute their drug programs
- Define the studies that must be conducted
- Decide the SEND IG and CT versions to be used and meeting FDA Business and CDISC Conformance Rules
- Responsible for regulatory submissions and defending regulatory questions and challenges
- Need to avoid meeting FDA Technical Rejection Criteria
- Control the process from planning to submission on a validated software as a service system without breaking capital budgets and incurring data management costs.
- Access Data Services at every stage by SEND specialists who keep abreast with the ongoing changes in standards, IG, CTs and Validation Rules.
Interested in moving forward? We request you to enter into an NDA confidentiality agreement.Mutual-NDA-Xbiom-SaaS-Clinical or send your standard NDA to regulatory@pointcross.com.
Pricing includes the following:
One time fee for set-up and online training
A single one-time charge of $4,000 for each unique StudyID managed in Xbiom Regulatory
- No limits on time, users or # of studies
- Minimum of 4 studies per year or $16,000 per year
Fixed priced Study Modeling, Data Standardization or quality checking services – quoted on request
No monthly subscription
For any optional services for study models, trial design, SEND merging or standardization services, quality checking of SEND against Study Reports, please fill out the form above to contact us or email us directly at regulatory@pointcross.com.

